Efficacy and safety of Femaxeen® for the treatment of urinary incontinence in women
This randomized, double-blind, placebo-controlled trial was conducted to investigate the efficacy and safety of Femaxeen® supplementation for the prevention and treatment of urinary incontinence (UI) symptoms.
To do so, 81 women with moderate, severe, or very severe urge, stress or mixed UI received either Femaxeen® (purified and specific cytoplasmic extracts of pollen 160 mg, pumpkin seed extract 300 mg and vitamin E 10 mg) or a placebo for 90 days.
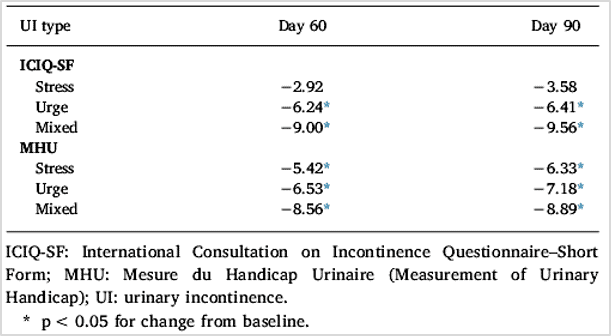
Results showed that Femaxeen® produced statistically significant improvements from baseline to Day 90 (p < 0.001 for all comparisons) in scores on the International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF), Measurement of Urinary Handicap (MHU) questionnaire, and Sandvik Incontinence Severity Index. Reduction from baseline in ICIQ-SF and MHU scores at Day 60 and Day 90 was significantly greater with Femaxeen® than placebo (p < 0.05 for all comparisons). Femaxeen® significantly reduced ICIQ-SF and MHU scores from baseline to Day 60 and Day 90 in all UI types (p < 0.05 for all comparisons except ICIQ-SF scores for stress UI). Femaxeen and placebo were well tolerated. Associated adverse events were few and mild in intensity.
Palacios S, Ramirez M, Lilue M, Vega B. Evaluation of Femaxeen® for control of urinary incontinence in women: A randomized, double-blind, placebo-controlled study. Maturitas. 2020;133:1–6.
