Sumac powder supplementation is beneficial for the management of non-alcoholic fatty liver disease
This randomized double-blind placebo-controlled clinical trial was conducted to assess the effects of sumac powder supplementation on hepatic fibrosis and some metabolic markers in patients with non-alcoholic fatty liver disease (NAFLD).
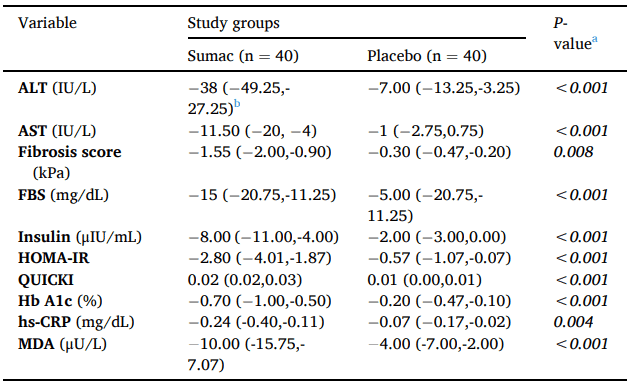
To do so, 80 patients with NAFLD were randomized to receive either 2000 mg/d of sumac powder or placebo for 12 weeks. Outcome measures were hepatic fibrosis and liver enzymes (ALT and AST) as well as fasting blood sugar (FBS), serum insulin, HbA1c, HOMA-IR (insulin resistance index), QUICKI (insulin sensitivity index), malondialdehyde (MDA), and high sensitivity C-reactive protein (hs-CRP).
Results showed that, after 12 weeks, subjects in the sumac group showed a greater decrease in hepatic fibrosis and liver enzymes as well as FBS, serum insulin, HbA1c, HOMA-IR, MDA, and hs-CRP, compared to the placebo (p < 0.05); while the QUICKI was significantly higher in the sumac group at the end of intervention.
In conclusion, sumac powder along with a low-calorie diet for 12 weeks was beneficial for the management of NAFLD.
Kazemi S, Shidfar F, Ehsani S, Adibi P, Janani L, Eslami O. The effects of sumac (Rhus coriaria L.) powder supplementation in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complement Ther Clin Pract. 2020 Nov 10;41:101259.
